PART 1 – organoid study
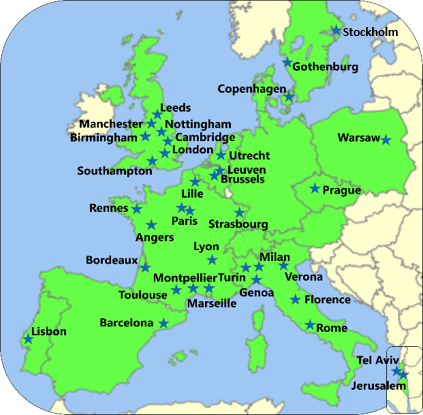
Between April 2018 and February 2020, biopsies were taken from 502 people with CF. 47 sites from 16 countries throughout Europe contributed to enroll people in HIT-CF, a huge effort:
Who participated?
People with CF who were 16 years of age* or older at the day of signing the informed consent for biopsy taking were enrolled in this European project. They have a confirmed diagnosis of CF and a rare genetic profile (see below: Is my genetic profile rare?). They made one visit to one of the participating hospitals to collect rectal biopsies for making their unique organoids.
*18 years or older in some countries because of national laws
Is my genetic profile rare?
Only patients with rare genotypes could participate in the HIT-CF Europe project. Patients with the following genetic profiles could not participate:
- one of the following mutations: F508del, G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, S549R, R117H, A455E, 3849+10kbC>T, or
- a combination of any two of the following mutations: G542X, 1717-1G>A, 621+1G>T, 3120+1G>A, 1898+1G->A, CFTRdele2,3 and 2183AA->G
What were additional inclusion and exclusion criteria?
Inclusion criteria -> patients participated if:
- They had a confirmed diagnosis of CF by their doctor. CF must have been diagnosed by the following:
– The new-born screen or one or more CF symptoms or sibling(s) with CF
– A sweat test result (SSC) higher than 60 mmol/L
Exclusion criteria -> patients could not participate in this project if:
- They had an additional disease (comorbidity) that increases the risk of administering a drug candidate would they be selected for a clinical trial. For example, they had severe liver disease. During their first visit for the project, the investigator decided whether they had a comorbidity that might put them at risk for this study.
- They have had a lung transplantation.
What is the timeline for this project?
People with CF could apply for participating in this project from March 2018 onwards in one of the participating CF-centers.
The subsequent laboratory tests of drug candidates on organoids was finished by end 2021.
Based on the responses in organoids, 52 patients will be selected for CHOICES. This clinical trial will start in the summer of 2022.
The HIT-CF project had to deal with severe delays because of the Covid-19 pandemic and because of important rearrangements within the pharmaceutical industry partners. You can find more information about this in our newsletters.
Additional important information
If you sign an additional consent, your mini-intestines will be stored in a biobank for future research. We will come back to you with more information on this in due course.
For more answers to your questions, see our FAQ.
Share this page: